OaCP – Fortress Diagnostics
CoViD-19 Total AB Serological Device
OaCP informs that the CoViD-19 Total AB Serological Device is not a domestic use kit and that the contents of this site are intended exclusively for healthcare professionals.
OaCP informa che CoViD-19 Total AB Serological Device non è un kit per uso domestico e che i contenuti di questo sito sono destinati esclusivamente agli operatori sanitari.
CoViD-19 Total AB Device
KIT for the detection of antibodies with the “Rapid” method (15 min).
Sample required: whole blood, serum or plasma
Product Characteristics
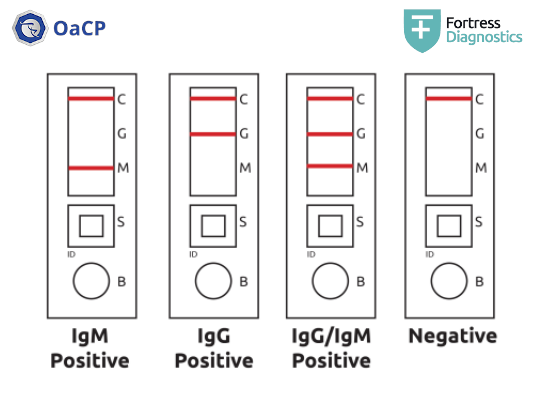
Qualitative test: indicates the presence both individually and simultaneously of specific IgG and IgM against COVID-19 (SARS-CoV-2)
High Specificity and Sensitivity: 95%
Anti-contamination: “one step” test.
Simultaneous detection of 2 classes of antibodies (IgG and IgM) + positive internal control
CE MARKED
CoViD-19 Total AB Device
KIT per la rilevazione degli anticorpi con metodo rapido (15 min).
Campioni richiesti: sangue, siero o plasma
Informazioni
Test qualitativo: indica singolarmente ma contemporaneamente la presenza degli anticorpi IgG ed IgM contro il COVID-19 (SARS-CoV-2)
Alta specificità e sensibilità: 95%
Anti-contaminazione: “one step” test.
Rilevazione simultanea di 2 classi di anticorpi (IgG and IgM) + controllo validità del test
CE MARKED
[hubspot type=form portal=9357629 id=0140551c-997e-4894-bb3c-97f84304ddaf]
The CoViD-19 Total AB Serological Device is registered among Italy’s Ministry of Health Database.
CoViD-19 Total AB Serological Device è un dispositivo registrato presso il Ministero della Salute.
World Health Organization (WHO) has defined our types of test within the best methods for the detection and identification of SARS-CoV-2 and variants.


